Electricity-driven chemistry enables a cleaner, controllable route to valuable nitrogen heterocycles for drug research.
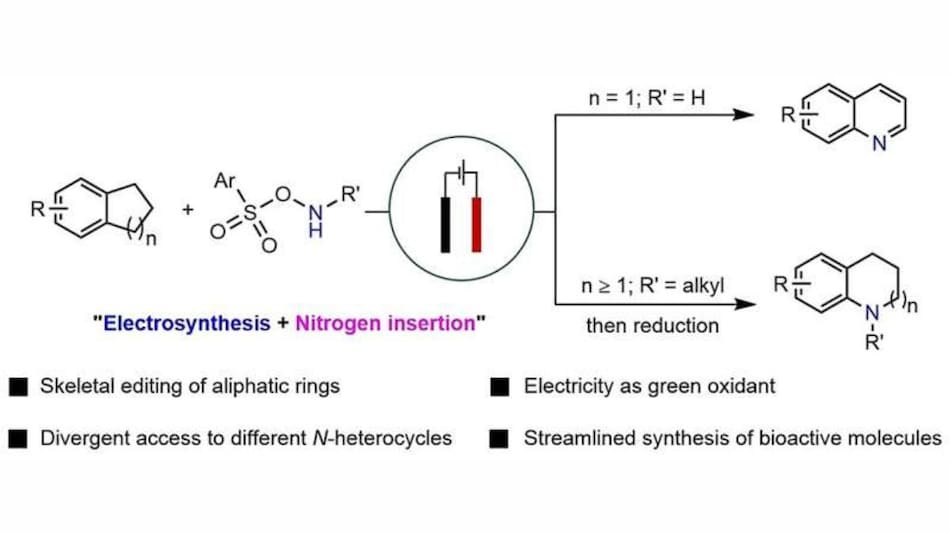
Electricity-driven reactions enable greener synthesis of nitrogen heterocycles used in drug development.
Click Here to Add Gadgets360 As A Trusted Source

Electricity-powered chemistry is providing a cleaner route to chemical Compounds containing nitrogen that are found in many of today’s pharmaceuticals. Scientists at the National University of Singapore have created a new process for inserting nitrogen atoms into difficult nitrogen-heterocycles, a stable carbon ring, using their special catalyst without taking too much time. The method is clean and waste-free, bypassing harmful chemicals that often accompany such changes and enabling almost pinpoint control over the resulting molecular structure. Silicon-containing compounds are common materials used in pharmaceuticals, agrochemicals, and advanced materials; hence, the development is important for a more sustainable drug design.
Electricity Enables Cleaner Nitrogen Insertion Into Drug-Ready Carbon Rings
According to a report published in Nature Synthesis, the research team led by Associate Professor Koh Ming Joo and Professor Zhao Yu demonstrated that electricity can drive nitrogen atom insertion into saturated carbocycles that are usually difficult to modify. The study explains how careful control of electrochemical conditions allows the same starting material to be converted into either functionalised quinolines or N-alkylated saturated nitrogen heterocycles, both of which are highly valued in medicinal chemistry.
Nitrogen heterocycles are essential building blocks in drug discovery, but making them often requires strong oxidising agents and produces large amounts of chemical waste. Directly breaking carbon–carbon single bonds to insert nitrogen has remained rare because these bonds are highly stable and resistant to reaction.
To overcome this issue, the NUS researchers employed electricity as a clean redox reagent. The reaction operates under ambient conditions and is highly tolerant to sensitive functional groups, which indicates that it can be used on complex molecules in a broad range without destroying them.
The researchers also demonstrated the method’s practical value by synthesising two potential ion-channel antagonist candidates. The strategy is now being trialled on other bioactive heterocycles, suggesting a wider application for electricity-driven synthesis in greener pharmaceutical production.